Concomitant expression and combined localization of Ets-1 an
作者:Zhao-Jiang Du, Peng Li, Yan-Nian Hui, Bai-Ren Wang, Xiao-Li Duan, Rui Zhang
【摘要】 To investigate the characteristics of Ets-1 and VEGF expression and distribution in the experimental diabetic rat retina. METHODS: Diabetes was induced by intraperitoneal injection of streptozotocin (STZ). At 4 weeks after STZ-injection, animals were sacrificed. Total proteins were isolated from retinas of experimental and control eyes and were assessed by Western blot analysis. Frozen cross sections of eyeballs with 14um thickness were used to perform double immunofluorescence staining with anti-Ets-1 and anti-VEGF antibodies. RESULTS: Both Ets-1 and VEGF expression were up-regulated in the diabetic retina, the distribution of Ets-1 and VEGF was identical to each other, and the two proteins were almost localized in all retinal layers. CONCLUSION: Ets-1 might contribute to the pathologic progress of the diabetic retina induced by VEGF.
【关键词】 Ets-1; VEGF; diabetic retina; angiogenesis
INTRODUCTION
Pathological growth of new blood vessels is the common final pathway in ocular neovascular diseases, such as, diabetic retinopathy, retinopathy of prematurity and age related macular degeneration, and often leads to catastrophic loss of vision. Ets-1 has been proved to be involved in angiogenesis of various tissues, including VEGF- and ischemia-induced retinal neovascularization [1]. Ets-1 belongs to a distinct class of sequence specific DNA-binding transcription factors, and receptor tyrosine kinases, such as, VEGF receptor 1 and VEGF receptor 2 which have been reported to have ETS-binding structures in their promoter regions [2,3].
VEGF has been proved to be a predominant angiogenic factor that mediates ocular neovascularization[4]. VEGF inhibition by soluble VEGF receptor 1 protein or adenovirus vector-encoding soluble VEGF receptor1 have been reported to reduce retinal neovacularization effectively [5,6].
Although the involvements of Ets-1 and VEGF in ocular neovascularization have been proved in the reports mentioned above, the relationship between expression of Ets-1 and VEGF in the diabetic retina remains to be determined. The present study was undertaken to analyze the expression and localization of Ets-1 and VEGF proteins in the experimental diabetic rat retina.
MATERIALS AND METHODS
Rabbit anti-Ets-1 polyclonal antibody was kindly provided by Doctor Jian (the Fourth Military Mediccal University). STZ was purchased from Sigma (St. Louis, MO). Anti-actin polyclonal antibody and mouse anti-VEGF monoclonal antibody were obtained from Santa Cruz Biotechnology, Inc. The BCA Protein Assay Kit was obtained from Beyotime (Beijing, China). All of the other agents were purchased from Sigma unless otherwise specified.
Animals All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Resolution on the Care and Use of Laboratory Animals and were authorized by the Fourth Military Medical University Bioethics Committee. Age-matched female Sprague- Dawley rats (obtained from animal centre of the Fourth Military Medical University) were used in all experiments. Rats were housed under a 12-hours light/dark cycle with free access to a standard rat food and water. Diabetes was induced by intraperitoneal injection of STZ (65mg/kg) [7,8], dissolved in sodium citrate buffer, pH 4.5. The control rats received equivalent volumes of buffer alone. Food was withdrawn 16 hours prior to the experiments. Rats were anesthetized with 100mg/kg of sodium pentobarbital injected intraperitoneally. STZ-injected rats were considered diabetic when exhibiting blood glucose levels of >13.9mmol/L(250mg/dL) within 3 days after diabetes induction. Each week rats were weighed and blood glucose (nondiabetic, 4 to 8mmol/L; diabetic, 13.9 to 27mmol/L) estimated using an Accutrend α glucometer (Mannheim Boehringer, NSW, Australia).
Western Blot Analysis Five rats of each group were sacrificed at 4 weeks after STZ injection to detect Ets-1 and VEGF expression. Total proteins from retinas were extracted with cold RIPA containing 20g/L protease inhibitors and supernatant fluid was collected after centrifuging at 10.000g for 10 minutes. The protein concentration of the supernatant was determined using the BCA Protein Assay Kit. Total proteins (50μg) were subjected to 100g/L polyacrylamide gel electrophoresis, and were transferred to nitrocellulose membrane (Schleicher & Schuell; Dassel, Germany). The blots were incubated overnight at 4° with primary antibodies followed by incubation for 2 hours with horseradish peroxidase-conjugated secondary antibody (1∶2 000 dilution) (Amersham International, Buckinghamshire, UK). Primary antibodies specific for Ets-1 and VEGF were used at 1∶500 and 1∶50 dilutions, respectively. Visualization was performed by enhanced chemiluminescence detection system (Amersham International).
Double Immunofluorescent Staining for Ets-1 and VEGF At 4 weeks after STZ injection, the rats were deeply anesthetized and perfused via cardiac puncture with 100mL physiological saline and subsequently with 400mL(40g/L) paraformaldehyde in 0.1mol/L phosphate buffer (PB, pH 7.4). After perfusion, the eyeballs were enucleated and postfixed in the same fixative for 2-4 hours before they were transferred into 0.1mol/L PB containing 200g/L sucrose and kept overnight at 4° Frozen cross sections of the eye were cut at 14μm thickness and mounted on gelatin-coated slides. All immunohistochemical procedures were carried out at room temperature.
Sections were blocked for 2 hours in phosphate buffered saline (PBS, pH 7.4), containing 50mL/L bovine serum albumin, 50mL/L normal goat serum and 10g/L Triton X-100. For double immunofluorescent staining, the sections were incubated with a mixture of rabbit anti-Ets-1 antibody (1∶100) and mouse anti-VEGF antibody (1∶150) diluted in a solution containing 0.01mol/L PBS (pH 7.4), 3g/L Triton and 10mL/L normal goat serum overnight at room temperature. After rinsing three times in PBS, the slides were then incubated with a mixture of goat anti-rabbit IgG-Alexa 488 (1∶400, Molecular Probes Inc, Eugene, OR, USA) and goat anti-mouse IgG-Texas Red (1∶600, Molecular Probes Inc) for 2 hours at room temperature, and then the slides were rinsed three times and coverslipped with 500mL/L glycerol in 0.01mol/L PBS. Then the fluorescent sections were observed and photographed with a confocal laser scanning microscope (CLSM, FV-300, Olympus, Tokyo, Japan).
Several controls were conducted for confirming the specificity of the primary antibodies. First, the omission controls were done. The primary antibodies were replaced by 0.01mol/L PBS (pH 7.4) containing 3g/L Triton and 10g/L BSA, and then incubated the sections with goat-anti-rabbit IgG-Alexa 488 and goat-anti-mouse IgG-Texas Red. Second, to ensure that there were no cross-reactivity between the primary antibodies and the non-related second antibodies in double fluorescent staining, some sections were incubated in goat-anti-rabbit IgG-Alexa 488 after the incubation in mouse anti-VEGF antibody, or in goat-anti-mouse IgG-Texas Red after the incubation in rabbit anti-Ets-1 antibody.
Statistical Analysis Determinations were performed in triplicate, and experiments were repeated at least three times. Results are expressed as the mean ±SD. The paired Student's t-test was used to evaluate the differences of Ets-1 and VEGF expression between control and experimental groups.
RESULTS
Rat Characteristics All diabetic rats gained significantly less weight than their age-matched control groups. The blood glucose values of the diabetic rats were significantly higher than control values for the entire length of the experiment (P <0.01). These data were similar with other previous reports [7-9].
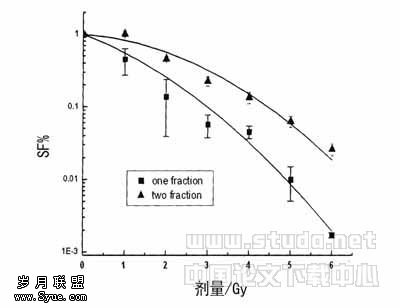
Expression of Ets-1 and VEGF Protein in the Diabetic Retina of STZ-induced Rat Model Western blot analysis was used to investigate Ets-1 and VEGF protein expression (Figure 1). Total proteins were isolated from retinas of rats at 4 weeks after STZ-injection (10 retinas from 5 rats of each group). Both Ets-1 and VEGF protein levels in the experimental eyes were significantly higher than that in the control eyes, reaching a 4- and 3.4-fold increase of control at the indicated time point (P <0.001), respectively.
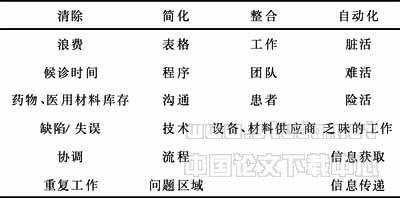
Localization of Ets-1 and VEGF in the Diabetic Rat's Retina We performed double immunofluorescence staining on cross sections of retina with anti-Ets-1 and anti-VEGF antibodies. The results demonstrated that the two proteins exhibited identical laminar distribution. Staining for both was strong in all layers of the diabetic retina. Both VEGF and Ets-1 immunoreactivity displayed typical Müller cells morphology, and were well localized to each other in Müller cells. At the same time, ganglion cells and RPE cells were also marked prominently by both anti-VEGF and anti-Ets-1 antibodies (Figure 2).
Figure 1 Upregulation of Ets-1 and VEGF expression in the experimental diabetic retina. Total proteins were isolated from retinas of experimental diabetic rats and from retinas of normal age-matched controls at 4 weeks after STZ-injection. Western blot analysis was performed using anti-Ets-1 antibody and anti-VEGF antibody A:Up-regulation of Ets-1 expression. B: Up-regulation of VEGF expression. Equal loading of proteins in each lane was confirmed by actin. Representative Western blots (top) and quantification (bottom). Results are shown as fold increase of control. Mean±SD of three independent experiments (each in triplicate). P <0.001.
Figure 2 Distribution of Ets-1 (green) and VEGF (red) in the diabetic retina A: Almost all retinal layers exhibited Ets-1 immunoreactivity. B: Almost all retinal layers exhibited VEGF immunoreactivity. C: A confocal image of Ets-1 and VEGF distribution. Both Ets-1 and VEGF immunoreactivity displayed typical Müller cells morphology, and were localized together in Müller cells. Ganglion cells and RPE cells were also marked prominently by both anti-Ets-1 and anti-VEGF antibodies. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment. Scale bar=200μm
DISCUSSION
Retinal neovascularization is a hallmark of proliferative diabetic retinopathy (PDR), and once it has happened, most of the damages due to it are irreversible. So it is much more important to prevent retinal neovascularization from developing in the pro-angiogenic stage than to cure it in the post-angiogenic stage. Therefore, the current study was aimed at identifying the characteristics of VEGF and Ets-1 expression and distribution in the early stages of experimental diabetic retina in order to find some clues to prevent diabetic retinal neovascularization. Our results indicated the followings: 1) both Ets-1 and VEGF expression were up-regulated in the early stages of experimental diabetic retina; 2) the distribution of VEGF and Ets-1 in the diabetic retina was identical to each other and they were localized together in all retinal layers.
In vivo , Ets-1 has been associated with new blood vessel formation under both physiological and pathophysiological conditions, and Ets-1 inhibition effectively suppressed retinal neovascularization in the ischemic retina [1,10-12]. Furthermore, it has been shown that angiogenic growth factors including vascular endothelial growth factor (VEGF), acidic fibroblast growth factor (FGF), and basic fibroblast growth factor (bFGF) induce Ets-1 through their receptors, such as, Flt-1 and KDR/Flk-1, and the Ets-1 functions as a principal regulator for angiogenesis that converts endothelial cells (ECs) into the angiogenic phenotype [13,14]. These data together with the alternations of VEGF and Ets-1 expression shown in our study indicated that Ets-1 was involved in the pathologic progress of the diabetic retina induced by VEGF.
The presumption was further confirmed by our results of double immunofluorescence staining with anti-Ets-1 and anti-VEGF antibodies which could demonstrate that the two proteins exhibited similar laminar distribution. VEGF has been reported to be secreted by many cell types in the retina, including pericytes, ganglion cells, Müller cells and RPE cells [15-17], which is similar to our results. However, the distribution of Ets-1 in the retina has not been studied until now. Our study for the first time showed that almost all retinal layers exhibited Ets-1 immunoreactivity and Ets-1 localized close together with VEGF in the diabetic retina.
Taken together, our data provided the first evidence of the retinal distribution of Ets-1 in the experimental diabetic rat retina. Our results also demonstrated that Ets-1 was similarly up-regulated with VEGF in the early stages of the diabetic retina, suggesting that Ets-1 might contribute to the pathologic progress of the diabetic retina induced by VEGF. This study might offer new insight into the mechanisms underlying diabetic retinal neovascularization and it suggested some new therapeutic clues to prevent the diabetic retinopathy.
【】
1 Watanabe D, Takagi H, Suzuma K, Suzuma I, Oh H, Ohashi H, Kemmochi S, Uemura A, Ojima T, Suganami E, Miyamoto N, Sato Y, Honda Y. Transcription factor Ets-1 mediates ischemia- and vascular endothelial growth factor-dependent retinal neovascularization. Am J Pathol ,2004;164(5):1827-1835
2 Wakiya K, Begue A, Stehelin D, Shibuya M. A cAMP response element and an Ets motif are involved in the transcriptional regulation of flt-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem ,1996;271(48):30823-30828
3 Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood , 2000;96(9):3078-3085
4 Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature ,1992;359(6398):843-845
5 Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A ,1995;92(23):10457-10461
6 Bainbridge JW, Mistry A, De Alwis M, Paleolog E, Baker A, Thrasher AJ, Ali RR. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther ,2002;9(5):320-326
7 Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest ,1998;102 (4): 783-791
8 Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes ,1998;47 (12):1953-1959
9 Glyn-Jones S, Song S, Black MA, Phillips AR, Choong SY, Cooper GJ. Transcriptomic Analysis of the Cardiac Left Ventricle in a Rodent Model of Diabetic Cardiomyopathy Molecular Snapshot of a Severe Myocardial Disease. Physiol Genomics ,2006;[Epub ahead of print]
10 Wernert N, Raes MB, Lassalle P, Dehouck MP, Gosselin B, Vandenbunder B, Stehelin D. c-ets1 proto-oncogene is a transcription factor expressed in endothelial cells during tumor vascularization and other forms of angiogenesis in humans. Am J Pathol ,1992;140(1):119-127
11 Hashiya N, Jo N, Aoki M, Matsumoto K, Nakamura T, Sato Y, Ogata N, Ogihara T, Kaneda Y, Morishita R. In vivo evidence of angiogenesis induced by transcription factor Ets-1: Ets-1 is located upstream of angiogenesis cascade. Circulation ,2004;109(24):3035-3034
12 Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest ,2005;115(9):2508-2516
13 Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci ,2000;902:201-207
14 Sato Y. Role of ETS family transcription factors in vascular development and angiogenesis. Cell Struct Funct ,2001;26(1):19-24
15 Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE.Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA ,1995;92(3):905-909
16 Guerrin M, Moukadiri H, Chollet P, Moro F, Dutt K, Malecaze F, Plouet J.Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol ,1995;164(2):385-394
17 Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA.Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol ,1995;113(12):1538-1544