血小板单采样品中残留白细胞计数两种方法的评价
作者:王拥军,沈卓岚,徐健,黄伯里,胡庆丰,冯武威
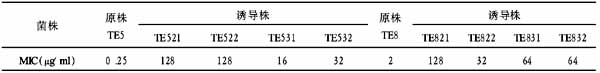
【摘要】 为评价流式细胞计数法和Nageotte血细胞计数法计数单采血小板中残留白细胞,应用系列的稀释实验研究其准确性;对已知白细胞浓度的同一样本,反复检测14次,研究其重复性;分别用流式细胞计数法和Nageotte血细胞计数法检测102份去白细胞的单采血小板样本,对其结果进行比较。结果发现,当白细胞浓度为0.8<WBC/μl<10时,两方法无显著差异(P>0.05)。结论: 两种方法均可用于少白细胞成分的质量控制。
【关键词】 血小板单采
Evaluation of Two Methods for Counting Residual White Blood Cells in Thrombocytaphoresis Concentrates
Abstract To evaluate flow-cytometry and Nageotte method for counting residual WBC in thrombocytaphoresis concentrates, their accuracies were determined by dilution studies separately;the repeatability was determined by measu-ring the interassay coefficient of variation for 14 replicates of a sample with known leukocyte concentration. 102 samples of leukocyte-depleted thrombocytaphoresis concentrates were detected by the above mentioned two methods,and the results were compared each other. The results showed that no difference was observed between two methods over a range of leukocyte concentrations from 0.8 to 10 WBC/μl(P>0.05). In conclusion,flow-cytometry and Nageotte methods can be used for quality control of WBC-reduced blood components.
Key words thrombocytapheresis; platelet; WBC; flow-cytometry method; Nageotte method
近年来,大力提倡和开展成分献(输)血,尤其是单采血小板。近代医学研究表明,血液中非性成分如白细胞等是一种“污染物”,去除单采血小板样品中白细胞的污染不仅可以降低非溶血性输血反应[1]、巨细胞病毒等嗜白细胞病毒的传播[2],还可以减少HLA同种异体免疫反应[3](血小板输注无效、移植物抗宿主病等)。国际上,已将引起HLA同种异体免疫反应的白细胞污染量控制在5×106以下[4]。这已远远超出了现有传统计数方法的检测限。近几年,已经报道了很多种低浓度白细胞的计数方法[5],其中Nageotte血细胞计数法和流式细胞计数法应用最广[6-8]。我们对这两种低浓度白细胞污染的计数方法进行了应用和评价。现将结果报告如下。
材料和方法
仪器与试剂
流式细胞计数:FACSCalibur流式细胞仪(BDIS产品) 、LeucoCOUNT试剂(BDIS产品) 和TruCOUNT磁珠管(BDIS产品)。Nageotte计数盘(Hausser Scientific Horsham,USA产品)。血小板单采机:MCS+ Haemonetics 与Spectra,COBE Laboratories。白细胞定值样本:由浙江大学医学院与浙江省人民提供。
样本收集
浙江省血液中心无偿献血者去白细胞单采血小板,共102份,留样后24小时内检测。
流式细胞计数
将100 μl样品加入到已知数量的TruCOUNT磁珠管中,再加入400 μl试剂,轻摇,室温暗置30分钟,混匀后计数 1.0×104磁珠中PI染色的白细胞数。利用以下公式白细胞浓度: WBC/μl=(白细胞数/104)×TruCOUNT总磁珠数/ 样品体积(100 μl)
Nageotte血细胞计数
样品5倍稀释,充池后加盖静置30分钟,普通光学显微镜计数1个大方格(50 μl)内白细胞总数(n)。利用以下公式计算白细胞浓度:
WBC/μl= (n/50)×5=n/10
统计学分析
实验结果以均数±标准差(X±D)表示,统计处理采用Microsoft Excel 软件,P<0.05认为有统计学意义。
结 果
准确性评价
已知白细胞浓度的样品通过1/10系列稀释(0.1-10.0 WBC/μl),每份标本分别用流式细胞计数法(A)和Nageotte血细胞计数法(B)平行检测。将测定值与预期值配对回归分析,得到相关系数(r)、斜率和截距。当0.1<WBC/μl<0.7时,流式细胞计数法的测定值和预期值呈直线相关,y=0.99x+0.01,r=0.99(P<0.001),Nageotte血细胞计数法测定值比预期值持续偏低,y=1.07x-0.21,相关性差,r=0.90(图1);当0.8<WBC/μl<10.0时,两方法的直线回归分别为A:y=1.01x-0.02,B:y=0.99x-0.07,测定值和预期值呈直线相关r=0.99(P<0.001),准确性高(图2)。
重复性评价
白细胞浓度为4.0/μl的同一样本,分别用两种方法重复检测14次,流式细胞计数法白细胞均值为4.07 /μl,95%可信区间为3.51-4.63/μl;Nageotte血细胞计数法白细胞均值为3.91/μl,95%可信区间为3.27-4.55/μl。两方法的14次检测结果均在95%可信区间内,重复性高(CV<10%)(表1)。Table 1. Interassay coefficient of variation for 14 replications of a sample with a mean leukocyte concentration of 4/μl(略)
应用性评价
分别用两种方法同时检测102份去除白细胞的单采血小板样品,流式细胞计数法检测白细胞均值为1.12/μl ;Nageotte血细胞计数法检测白细胞均值为1.10/μl,所有检测结果均在95%可信区间内。经统计分析,发现两方法无显著差异(P>0.05),结果见表2。Table 2. Comparison of results obtained by flow cytometry and Nageotte method (略)
讨论
由于白细胞去除技术的不断,低浓度白细胞检测也面临更新的挑战,传统的全自动血细胞计数仪检测精度>100/μl[9,10];Neubauer计数池精度为2.8-5.6/μl;Nageotte计数池与流式仪的精度均可达到0.05-0.1/μl,当白细胞数<0.8/μl,Nageotte血细胞计数法和流式细胞计数法一样,其测定值和预期值呈直线相关(图2),准确度高、重复性好(CV<10%),检测102份样本,两者并无显著差异。基于以上结果,本研究肯定了Nageotte血细胞计数法和流式细胞计数法在相应检测限内高度的准确性和可重复性。Wenz[10]与Kao等[11]也发现,Nageotte血细胞计数明显比传统方法准确可靠,与流式细胞计数相比,具有不需要特殊的仪器和试剂、简单、快速、等优点,适合于血站系统血液成分白细胞污染的常规质量控制[12]。
【】
1Wenz B,Gurtlinger KF,O′ Toole AM,et al. Preparation of gra- nulocyte-poor red cells by microaggregate filtration:a simplified method to minimize febrile transfusion reaction. Vox Sang,1980; 39: 282-287
2Gilbert GL,Hayes K,Hudson IL,et al. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leucocytes. Neonatal Cytomegalovirus Infection Study Group. Lancet,1989; 1: 1228-1231
3Sniecinski I,O′ Donnell MR,Nowicki B,et al. Prevention of refractoriness and HLA-alloimmunization using filtered blood pro- ducts. Blood,1988; 71: 1402-1407
4Fisher M,Chapman JR,Ting A,et al. Alloimmunization to HLA antigens following transfusion with leukocyte-poor and purified platelet suspensions. Vox Sang,1985; 49: 331-335
5Dzik S. Principles of counting low numbers of leukocytes in leuko reduced blood components.Transfus Med Rev,1997; 11: 44-55
6Lutz P,Dzik WH. Large volume hemocytometer chamber for accurate counting of white cells(WBCs) in WBC-reduced platelets:validation and application for quality control of WBC- reduced platelets prepared by apheresis and filtration. Transfusion,1993; 33: 409-412
7Masse M,Naegelen C,Pellegrini N,et al. Validation of a simple method to count very low white cell concentrations in filtered RBCs or platelets,Transfusion,1992; 32: 565-571
8Vengelen-Tyler V. Technical manual. Bethesda: American Association of Blood Banks. 1996: 722-725
9Kjeldsberg CR,Knight JA. Body fluids: laboratory examination of amnionic,cerebrospinal,seminal,serous,and synovial fluids. In Laboratory methods. Chicago: American Society of Clinical Pathologists. 1986: 154-155
10Wenz B,Besso N. Quality control and evaluation of leukocyte-depleting filters. Transfusion,1989; 29: 186-187
11Kao KJ,Scornik JC. Accurate quantitation of the low numbers white cells in white cell-depleted blood components. Transfusion,1989; 29: 774-778
12Rebulla P,Dzik WH. Multicenter evaluation of methods for counting residual white cell in white cell-depleted red blood cells. The Biomedical Excellence for Safe Transfusion (BEST) Working Party of the International Society of Blood Transfusion. Vox Sang,1994; 66: 25-32